
Page 41
December 9-10, 2019 | Barcelona, Spain
Volume 14
ARTHRITIS AND RHEUMATOLOGY
ANATOMY AND PHYSIOLOGY
13
th
International Conference on
3
rd
International Conference on
&
Journal of Orthopaedics Trauma Surgery
and Related Research
Rheumatology Congress 2019 & Anatomy and Physiology 2019
December 09-10, 2019
J Orthop Trauma Surg Rel Res, ISSN: 1897-2276
Inflammatory back pain in psoriatic arthritis is significantly more responsive to
corticosteroids compared to back pain in ankylosing spondylitis: A prospective, open-
labelled, controlled pilot study
Muhammad Baig
Galway University Hospital, Ireland
Background
: The efficacy of corticosteroids in patients with psoriatic
arthritis (PsA) and inflammatory back pain has not been studied to date. In
this controlled trial, we aimed to investigate the comparative performance
of corticosteroids in patients with active axial-PsA (AxPsA) versus those
with active ankylosing spondylitis (AS).
Methods
: Patients with AxPsA and AS (naïve to biologic therapies), who
not only had clinically active disease, but also had bone marrow oedema
on magnetic resonance imaging of the sacroiliac joints, were recruited.
Clinically active disease was defined as inflammatory back pain (fulfilling
Assessment of Spondyloarthritis International Society (ASAS) expert
criteria), with spinal pain score (numerical rating scale 0-10) ≥4 and Bath
AS Disease Activity Index (BASDAI) score ≥4 despite taking nonsteroidal
anti-inflammatory drugs. Moreover, we recruited a control group of patients with non-inflammatory lower back pain. All patients
received a single, intra-muscular dose of depot corticosteroid injection (triamcinolone acetonide 80 mg) at baseline. The intra-
muscular corticosteroid option was used to overcome any drug compliance issues. Clinical outcome assessments were made
at the following time points: baseline, week 2, and week 4. The primary efficacy end point was mean change in Ankylosing
Spondylitis Disease Activity Score (ASDAS) at week 2. Key secondary outcomes were mean change in the BASDAI, Bath
Ankylosing Spondylitis Functional Index (BASFI) and Ankylosing Spondylitis Quality of Life (ASQoL) at weeks 2 and 4.
Results
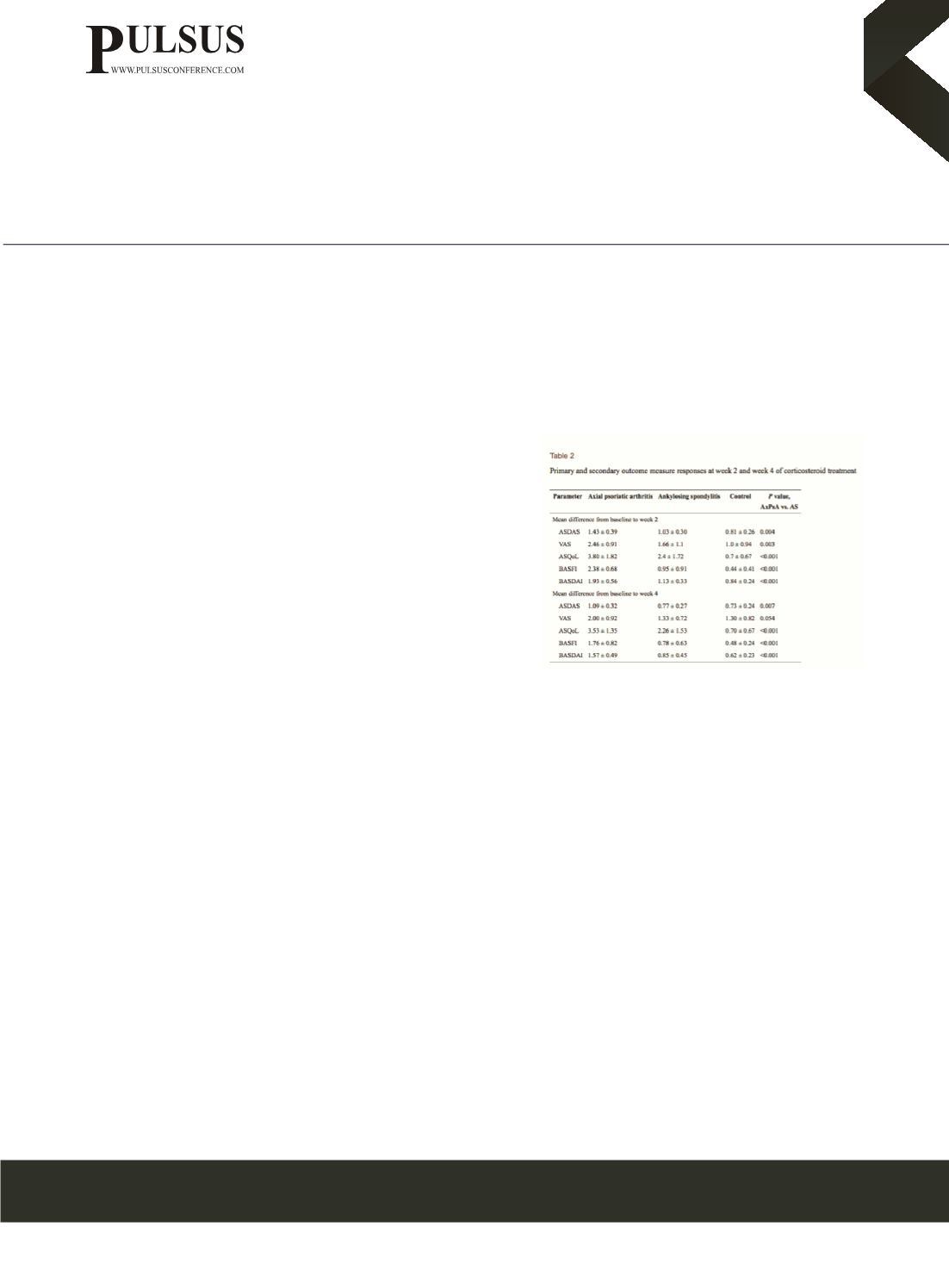
: In total, 40 patients were recruited (15 with AxPsA, 15 with AS, and 10 controls). At week 2 following corticosteroid
treatment, patients with AxPsA had significantly greater improvement in the mean ASDAS compared to patients with AS (1.43
± 0.39 vs. 1.03 ± 0.30, p = 0.004), and also when compared to controls (p <0.001). At week-4, similar significant trend of
ASDAS improvement was seen among AxPsA patients compared to AS patients (1.09 ± 0.32 vs. 0.77 ± 0.27, p = 0.007) and
controls (p < 0.001). Similarly, the mean BASDAI, visual analogue scale spinal pain score, ASQoL and BASFI improved
significantly among patients with AxPsA compared to patients with AS and controls at week 2 (p <0.05), with this trend also
largely maintained at week 4.
Conclusions
: Axial inflammation in patients with PsA responds significantly better to corticosteroids than in patients with AS.
This furthers the argument and adds to the growing evidence that AxPsA and AS are distinct entities.
nouman142@gmail.com















