
Page 28
December 9-10, 2019 | Barcelona, Spain
Volume 14
ARTHRITIS AND RHEUMATOLOGY
ANATOMY AND PHYSIOLOGY
13
th
International Conference on
3
rd
International Conference on
&
Journal of Orthopaedics Trauma Surgery
and Related Research
Rheumatology Congress 2019 & Anatomy and Physiology 2019
December 09-10, 2019
J Orthop Trauma Surg Rel Res, ISSN: 1897-2276
Switching from reference to biosimilar rituximab in rheumatoid arthritis patients:
Experience from a single rheumatology centre
Renna Daniela
University of Bari, Italy
Statement of the problem
: Clinical and real-word data on the effects of switching are currently limited to transition studies of
approved biosimilars. Few data have been published about the outcome of switching from reference to biosimilar rituximab in
rheumatoid arthritis (RA) and this monocentric study aimed to evaluate the effectiveness and safety of this switching.
Material and methods
: we evaluated RA patients who consented to switch to biosimilar RTX with a 52-weeks follow-up.
Clinical and laboratory findings, DAS28, CDAI and SDAI, as well as any adverse events (AEs) have been recorded, before the
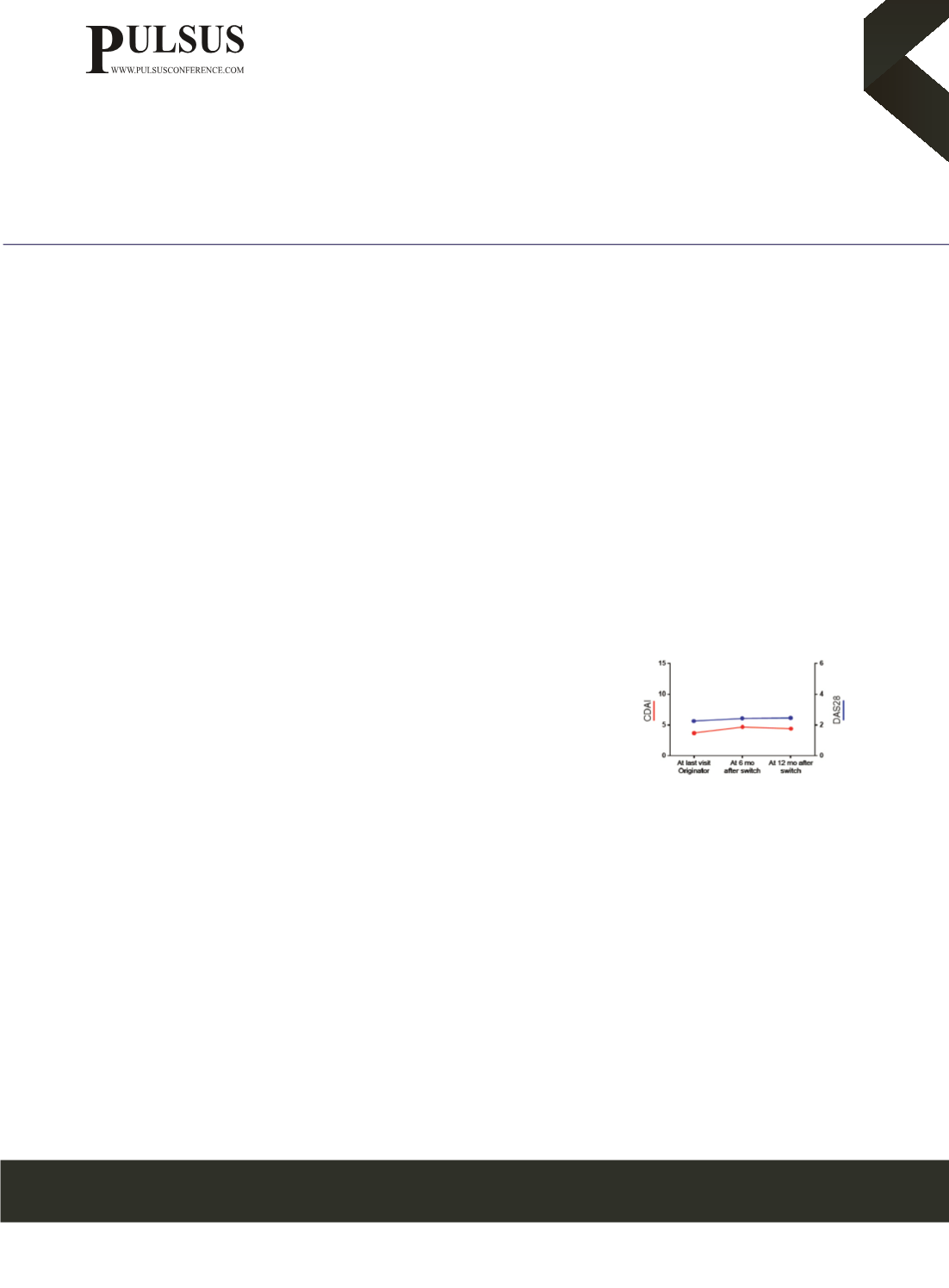
first dose and 6 and 12 months after the switch. Results: a total of 62 RA patients consented to switch after a median (IQR) of 12
(6-14) cycles of reference RTX. At last follow-up visit of reference RTX a mean of 2,2 ± 1,0, 3,7 ± 4,3 and 4,2 ± 4,4 of DAS28,
CDAI and SDAI, respectively, were observed. After switching to biosimilar RTX no statistically significant changes in DAS28,
CDAI and SDAI were observed. At 12months after switching to biosimilar RTX, 50 (81%), 54 (87%) and 56 (90%) patients
were still on remission/low-disease activity according to DAS28, CDAI and SDAI, respectively (p > 0,05). In biosimilar RTX
we registered 3 cases of leukopenia, 9 infections and 5 hospitalizations. All cases were
resolved after RTX suspension and specific antibiotic treatment. These AEs occurred after
a global exposure to 11 (4-11) cycles of RTX in biosimilar RTX. Similar conditions have
been already observed during treatment with reference RTX (3 leukopenia; 15 infections;
3 hospitalizations) after 8 (5-12) cycles.
Conclusion
: our study demonstrates the maintenance of effectiveness of biosimilar after
switching from reference RTX. On safety concerns are more relevant the cumulative and
repeated administrations of RTX rather than the use of the biosimilar.
Biography
Daniela Renna graduated in December 2016 in Medicine and Surgery magna cum laude at the University of Bari. She is a Rheumatology
resident in Bari University, currently working at the Rheumatology Unit of Bari’s Policlinico. She spent her first year of residency at
the Rheumatology ward; she’s currently attending her second year of residency at the outpatient clinic “pre-infusional visits”; she
manages follow-up visits of patients with RA, SpA and other rheumatic diseases in treatment with i.v. biological therapies (Tocilizumab,
Abatacept, Infliximab, Rituximab).
daniela.renna.91@gmail.com















