Osteoid osteoma: The results of conventional open excision
Received: 11-Dec-2020 Accepted Date: Jan 07, 2021 ; Published: 25-Jan-2021, DOI: 10.37532/1897-2276.2021.16(1).3
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Conventional (en bloc) open excision was the standard treatment for osteoid osteoma. With the advancement of radiological techniques, percutaneous procedures with less morbidity have been introduced. The clinical outcomes and risk for complications of the conventional (en bloc) open excision will be discussed. Patients and methods: Twenty-one patients with osteoid osteoma were prospectively treated with conventional (en bloc) open excision between January 2012 and June 2017. The clinical findings and radiological investigations as well as the operative data for all patients were recorded. Radiological assessment was based mainly on plain X-rays. CT scan was performed in seven patients and a bone scan in three patients. Their mean age was 17.7 years (range 15-25) and mean follow-up was 2.2 years (range 1-3). All were males. Osteoid osteoma was located in the lower limb in 20 patients, and in the upper limb in one patient. In all patients, plain X-rays were used intraoperatively before closure to confirm complete excision. The removed bone was routinely sent for histological examination. Results: Improvement of pain intensity was documented in 16 patients during the first week postoperatively. In five patients, improvement came gradually during the first 6 months postoperatively. That was attributed to the extensive bone curettage rather than the remaining nidus. Three patients had increased pain in the anterior superior iliac spine from the bone-graft harvesting area. One patient developed a partial foot drop. The tumor was in the proximal fibula and neurapraxia of the lateral popliteal nerve developed due to compression by postoperative hematoma and was successfully treated conservatively. No patient developed tumor recurrence. No pathological fracture or wound infection occurred. Conclusions: Open en bloc excision of osteoid osteoma has the risk of increased morbidity and complications. It is recommended when hospital facilities and equipment are unavailable to perform percutaneous excision.
Keywords
osteoid osteoma, open excision, benign tumors
Introduction
Osteoid osteoma is the third most common benign neoplasm of bone, occurring predominantly in adolescents and young adult male patients with an approximate male/female ratio of 2 to 1 [1]. It is a small, distinctive, non-progressive, benign osteoblastic lesion that is usually accompanied by severe pain. In 1935 Jaffe was the first to report the identification of this osteoblastic lesion [2].
Osteoid osteoma may occur in any bone but predominantly occur in the appendicular skeleton. According to the Musculoskeletal Tumor Society Staging System for benign tumors, osteoid osteoma is a stage-2 lesion. It is classified as cortical, cancellous, or subperiosteal. Cortical lesions are the most common [3]. In over 50% of cases, the lesions are centered on the cortex of femoral and tibial diaphysis [4].
In long bones, osteoid osteoma is more often situated in the corticodiaphyseal or metaphyseal regions, but other localizations such as intramedullary, subperiosteal, epiphyseal, or apophyseal have also been noted [5].
Pain is the most common clinical presentation. It tends to become increasingly severe at night and is usually relieved by salicylates [6]. Its clinical diagnosis is confirmed with the radiological appearance of a small radiolucent area, known as ‘‘nidus,’’ equal to or less than 1 cm surrounded by a thick zone of sclerotic bone. Osteoid osteoma treatment varies from conservative to operative, from wide excision of the nidus [6] to percutaneous CT-guided core-drill excision [7], destruction of the nidus using radio-frequency thermo-coagulation [8], laser [9], or ethanol injection [10]. However, complete surgical excision, without complementary therapies, was the gold standard for the treatment of osteoid osteoma.
The purpose of this study was to discuss the clinical outcomes and morbidity associated with conventional (en bloc) open excision in the treatment of osteoid osteoma.
Patients and Methods
Twenty-one patients with osteoid osteoma were diagnosed and prospectively treated with conventional (en bloc) open excision between January 2012 and June 2017. The diagnosis was established from their history, clinical examination, and radiological investigations. Radiological assessment was based mainly on plain X-rays. CT scan was performed in seven patients and a bone scan in three patients.
The clinical findings and radiological investigations as well as the operative data for all patients were recorded. Their mean age was 17.7 (range 15-25) years and mean follow-up was 2.2 (range 1-3) years. All were males and written consent was taken from every patient before the start of the study.
Seventeen patients had moderate-to-severe pain, and in 15, there was night pain that disturbed their sleep. The diagnosis was established with an average 13.5 months delay from the presentation of symptoms (range 5 months to 2 years).
Of the 21 patients, 20 patients had the tumor located in the lower extremities, which included 3 cases with the tumor in the proximal femoral metaphysis, 2 in the femoral neck, 5 in lesser trochanter, 2 in the femoral diaphysis, 2 in the proximal tibia, 2 in the tibial diaphysis, 3 in the distal tibia, and 1 in the proximal fibula. One patient had the tumor located in the upper extremity which was in the radial styloid process as in Table 1.
| Case | Age (Year) | Sex | Symptoms |
Duration of symptoms (month) |
Location |
Follow up (month) |
|---|---|---|---|---|---|---|
| 1 | 17 | M | Hip pain | 6 | L lesser trochanter | 20 |
| 2 | 14 | M | Knee pain | 24 | R proximal fibula | 22 |
| 3 | 23 | M | Hip pain | 8 | R lesser trochanter | 18 |
| 4 | 19 | M | Knee pain | 12 | L proximal tibia | 30 |
| 5 | 16 | M | Ankle pain | 24 | L distal tibia | 12 |
| 6 | 15 | M | Hip pain | 7 | R femoral neck | 34 |
| 7 | 15 | M | Thigh pain, limping | 24 | L femoral diaphysis | 28 |
| 8 | 14 | M | Knee pain | 5 | L proximal tibia | 22 |
| 9 | 18 | M | Hip pain | 6 | L femoral neck | 36 |
| 10 | 20 | M | Thigh pain, limping | 12 | L femoral diaphysis | 24 |
| 11 | 16 | M | Thigh pain | 24 | R proximal femoral metaphysis | 30 |
| 12 | 20 | M | Wrist pain | 12 | R radial styloid process | 30 |
| 13 | 15 | M | Hip pain, limping | 24 | R lesser trochanter | 33 |
| 14 | 17 | M | Ankle pain | 6 | L distal tibia | 25 |
| 15 | 16 | M | Hip pain, limping | 5 | R lesser trochanter | 28 |
| 16 | 22 | M | Ankle pain | 8 | R distal tibia | 30 |
| 17 | 14 | M | Thigh pain | 12 | L proximal femoral metaphysis | 32 |
| 18 | 15 | M | Hip pain, limping | 10 | R lesser trochanter | 34 |
| 19 | 21 | M | Thigh pain | 12 | L proximal femoral metaphysis | 36 |
| 20 | 18 | M | Lower leg pain | 24 | R Tibial diaphysis | 33 |
| 21 | 23 | M | Lower leg pain | 20 | R Tibial diaphysis | 35 |
Table 1 Patient demographics
Overall, bone grafts were used in 12 patients: autografts from the iliac spine in eight and synthetic bone graft material in the remaining 4 patients. In four patients who had osteoid osteoma, 2 in the femoral shaft, and 2 in the proximal tibia, after excision, internal fixation was applied with bone grafting.
In all patients, plain X-rays were used intraoperatively before closure to confirm complete excision. The removed bone was routinely sent for histological examination. Perioperatively, all patients had prophylactic i.v. antibiotics. Postoperative protections (such as crutches, or casting) were usually administrated.
Patients were followed up every 4 weeks in the first 3 months to evaluate bone healing, residual symptoms, and potential complications. Then follow-up was at 3-month intervals. Complete relief of pain and union of the original tumor site at a minimal 1-year follow-up were considered as curative. Then the patients were followed-up once every year until the end of the follow-up period.
Results
Substantial improvement regarding pain intensity was documented in 16 patients during the first week postoperatively. In five patients, improvement came gradually during the first 6 months postoperatively. These patients complained about a ‘‘different type of pain’’ compared to the pain they had preoperatively. That was attributed to the extensive bone curettage rather than the remaining nidus. Another three had increased pain in the anterior superior iliac spine from the bone-graft harvesting area.
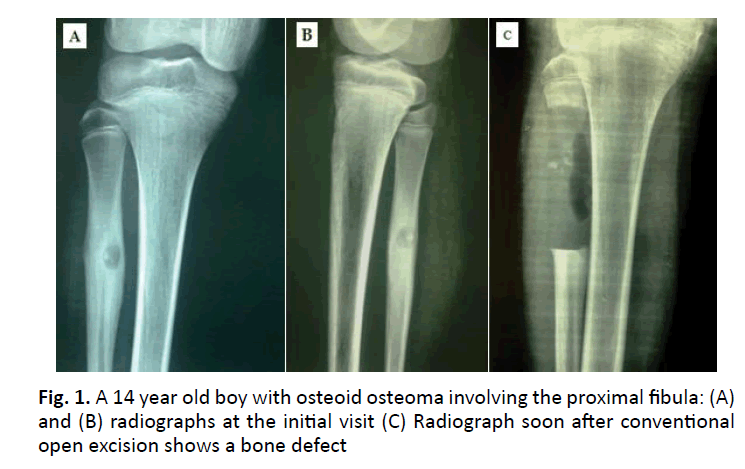
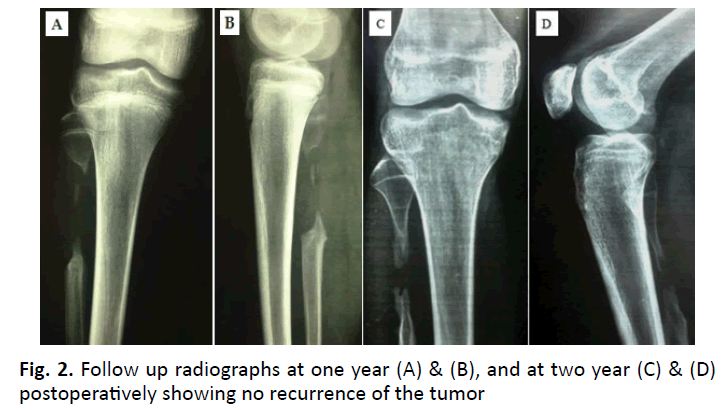
One patient developed a partial foot drop (case 2). The tumor was in the proximal fibula (Fig. 1 and 2). A sizable postoperative hematoma that compressed the lateral popliteal nerve as well as careless retraction intraoperatively resulted in neurapraxia of the nerve. That was confirmed by a nerve conduction study. Then the patient was treated conservatively by a splint for the foot drop, anti-edematous, and NSAID medications until the hematoma subsided and gradually the neurapraxia of the nerve has been cured. No patient developed tumor recurrence. No pathological fracture or wound infection occurred.
Discussion
The natural history of an untreated osteoid osteoma is natural regression, which occurs within 6 to 15 years but can be reduced to 2 to 3 years with treatment with aspirin or other NSAIDs [11]. Nonoperative management should be considered in patients where osteoid osteoma is not easily accessible by surgery [12]. However, the side effects of prolonged medication and the lack of histological diagnosis are still a major concern in conservative treatment [13].
Generally, the treatment aiming to destroy the nidus varies depending on the location of the lesion, experience of the surgeon, and facilities of the hospital. Complete surgical excision, without complementary therapies, was the treatment of choice for osteoid osteoma, with a low recurrence rate. The conventional method is the en bloc open wide excision (removing the nidus with a surrounding bone block) [2,6]. After exposure, the sclerotic bone covering the nidus is removed layer by layer with chisels. Sometimes, when removing reactive bone, one little vessel can be found. It is perforating the cortex from the periosteum to the nidus and can be used as a guide for the surgeon to find the nidus [2].
Pain regresses immediately after surgery. If pain remains unchanged or there is only partial relief, this suggests that the nidus has not been excised or destroyed. If the pain returns after a period of a few months to a year, then the nidus has been subtotally removed. The imaging techniques will reveal the presence of the nidus. Recurrence never occurs after the removal or destruction of the nidus. This was proved to be true in the current study as no recurrence occurred in any case as the nidus was excised in all cases.
Yildiz et al., [14] treated 104 symptomatic patients operatively with either wide resection or curettage and reported a success rate of 86.7% with an average follow-up of 2.5 years. In another series, incomplete nidus resection has been linked with local recurrence [15].
However, alternative options using a less invasive approach and exploiting technology have been developed to minimize potential complications associated with the more substantial operative tissue damage during wide excision of the nidus. Good results have been reported using percutaneous drill resection under CT control [7], cryo treatment or radiofrequency ablation [8], thermal destruction using laser photocoagulation [9], and drill resection with subsequent injection of ethanol [10].
However, most patients in the literature undergoing percutaneous ablation or resection required general anesthesia for pain control. The need for a general anesthetic increases the invasive nature and the cost of these procedures and reduces the advantages of percutaneous treatment over surgical resection. Furthermore, these techniques require equipment not commonly available in all hospitals [16].
Roger et al., [17] reported 16 patients who were treated using percutaneous CT-guided excision and had satisfactory results in 14 patients. Muscolo et al., [18] reported superior outcomes of CT-guided minimally invasive surgery rather than open surgery. Petrilli et al., [19] have evaluated computed tomography-guided percutaneous trephine removal of the nidus in 18 cases of osteoid osteoma, demonstrating that this is a safe and effective method for surgical resection of the lesion with reduced hospitalization time and less postoperative pain.
In conventional (en bloc) open excision, the nidus is exposed and then the en block is curetted or removed [7, 20]. If the nidus is removed completely, the risk of recurrence is eliminated. The reason for recurrence is the incomplete excision of the nidus [15]. However, it is difficult to identify and localize the nidus at the time of surgery. For symptomatic relief, the entire nidus has to be excised. Complete removal of the sclerotic reactive bone, however, is not required. Preoperative roentgenograms and CT scans delineate the location of the nidus [21].
This resection has the drawback of an open surgical approach with excision of sclerotic bone wider than what it should be, leaving behind a bone defect which may require bone grafting and internal fixation depending on the size of the bone defect left by the resection with consequent restrictions on postoperative activities and weight-bearing. Furthermore, this increases the patient’s morbidity and often requires a second procedure to remove the metalwork [22]. This was found to be true in the current study as bone grafts were used in 12 patients. Furthermore, internal fixation was applied in addition to bone grafting in four patients who had osteoid osteoma in the midshaft of the femur and proximal tibia. All these are factors resulted in increased hospital stay and cost as well as more morbidity.
Unroofing and curettage have a role in structurally critical locations, such as the neck of the femur because the central sclerotic structure is not disrupted. This was found to be true in the current study as we had 2 cases of osteoid osteoma in the femoral neck treated by unroofing and curettage.
The major complication in the current study was a case of compression neurapraxia of the lateral popliteal nerve after resection of osteoid osteoma in a proximal fibula. The neurapraxia was caused by careless intraoperative retraction and aggressive resection of the nidus and the surrounding sclerotic bone, which caused a sizable hematoma. Then the patient was treated conservatively by a splint for the foot drop, antiedematous, and NSAID medications until the hematoma subsided and gradually the neurapraxia of the nerve has been cured.
On the other hand, most minimally invasive techniques require special instruments, facilities, and medical expertise. The advantages of such techniques include the use of fine instruments, a more ‘‘kind’’ surgical approach, and removal of less bone. It is reported that the techniques result in reduced cost, shorter hospital stay and rehabilitation, faster return to work activities, and lower risk of associated complications, morbidity, and recurrence rate. They can be performed as outpatient procedures and sometimes are carried out under local anesthesia. Also, they appear to be particularly suited to deep sites, such as in the neck of the femur and pelvis [23, 24]. Furthermore, intra-articular osteoid osteoma was reported to be removed arthroscopically [25].
However, the minimally invasive techniques are not indicated in most cases of osteoid osteoma of the spine, in close anatomical relationship to dural and neural structures [6], in osteoid osteoma of small bones, or cases of recurrent lesions. They should also be avoided when the lesion is close to a neurovascular bundle and in those more than 1 cm across in which multiple perforations and supplementary percutaneous curettage should be used. There is also the disadvantage of the lack of histological confirmation in most cases [2].
Regardless of the technique used, complete removal or destruction of the nidus is necessary to obtain a successful outcome [20]. A biopsy must be taken at the time of intervention to confirm the diagnosis. This study has some limitations regarding the small sample size. A greater number of patients would help to validate the conventional open excision for the treatment of osteoid osteoma.
Conclusion
Conventional (en bloc) open excision of osteoid osteoma prevents recurrence and leads to substantial improvement in pain intensity during the first week postoperatively. This can prevent the need for a second operation if the tumor is incompletely removed by minimally invasive techniques. Furthermore, surgery is still performed in instances where the location of the lesion precludes percutaneous techniques.
However, this technique is associated with increased morbidity and higher risk for complications compared to more minimally invasive procedures that have shown promise with highly successful outcomes.
We recommend en bloc surgical excision of osteoid osteoma when hospital facilities and equipment are unavailable to perform percutaneous excision.
Disclosure of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- Schajowicz F. Bone Forming Tumors. In: Tumors and Tumor-Like Lesions of Bones and Joints. New York. Springer 1981; 61:36-47.
- Jaffe H.: Osteoid osteoma: A benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg. 1935; 31:709-728.
- Kitsoulis P., Mantellos G., Vlychou M.: Osteoid osteoma. Acta Orthop Belg. 2006; 72:119-125
- Cakar M., Esenyel CZ., Seyran M., et al.: Osteoid osteoma treated with radiofrequency ablation. Adv Orthop. 2015; 2015:807274
- Ghanem I.: The management of osteoid osteoma: Updates and controversies. Curr Open Pediatr. 2006;18:36-41.
- Campanacci M., Ruggieri P., Gasparini A., et al.: Osteoid osteoma. Direct visual identiï¬cation and intralesional excision of the nidus with minimal removal of bone. J Bone Joint Surg Br 1999;81:814-820
- Buhler M., Binkert C., Exner G.: Osteoid osteoma: Technique of computed tomography-controlled percutaneous resection using standard equipment available in most orthopedic operation rooms. Arch Orthop Trauma Surg. 2001;121:458-461
- Lindner NJ., Ozaki T., Roed R., et al.: Percutaneous radiofrequency ablation in osteoid osteoma. J Bone Joint Surg Br. 2001;83:391-396
- Witt JD, Hall-Cruggs MA, Ripley P, et al.: Interstitial laser photocoagulation for the treatment of osteoid osteoma. J Bone Joint Surg Br. 2000;82:1125-1128
- Duda SH., Schnaatterbeck P., Harer T., et al.: Treatment of osteoid osteoma with CT-guided drilling and ethanol instillation. Dtsch Med Wochenschr. 1997;122:507-510
- Moberg E.: The natural course of osteoid osteoma. J Bone Joint Surg Am. 1951;33:166-170.
- Carpintero-Benitez P., Aguirre MA., Serrano JA., Lluch M.: Effect of rofecoxib on pain caused by osteoid osteoma. Orthopedics. 2004;27:1188-1191.
- Kneisl JS., Simon MA.: Medical management compared with operative treatment for osteoid osteoma. J Bone Joint Surg Am. 1992;74:179-185.
- Yildiz Y., Bayrakci K., Altay M., et al.: Osteoid osteoma: The results of surgical treatment. Int Orthop. 2001;25:119-122.
- Regan MW., Galey JP., Oakeshott RD.: Recurrent osteoid osteoma: Case report with ten year asymptomatic interval. Clin Orthop. 1990;253:221-224.
- Rajasekaran S., Karthik K., Chandra VR., et al.: Role of intraoperative 3D C-arm-based navigation in percutaneous excision of osteoid osteoma of long bones in children. J Pediatr Orthop B. 2010;19:195-200.
- Roger B., Bellin MF., Wioland M., et al.: Osteoid osteoma: CT-guided percutaneous excision confirmed with immediate follow-up scintigraphy in 16 outpatients. Radiology. 1996;201:239-243.
- Muscolo DL., Velan O., Pineda Acero G., et al.: Osteoid osteoma of the hip. Percutaneous resection guided by computed tomography. Clin Orthop Relat Res. 1995;310:170-175.
- Petrilli M., Senerchia AA., Petrilli AS., et al. Computed tomography-guided percutaneous trephine removal of the nidus in osteoid osteoma patients: experience of a single center in Brazil. Radiol Bras. 2015;48:211-215.
- Dahlin DC., Unni KK.: In: Bone tumors: General aspects and data on 8542 cases, 4th ed, Thomas, Springï¬eld 1987;pp. 88- 101.
- Frassica FJ., Waltrip RL., Sponseller DD., et al.: Clinico-pathologic features and treatment of osteoid osteomas and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27:559-574
- Atesok KI., Alman BA., Schemitsch EH., et al.: Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19:678-89.
- Karagöz E, Özel D, Özkan F, et al:. Effectiveness of computed tomography guided percutaneous radiofrequency ablation therapy for osteoid osteoma: Initial results and review of the literature. Polish J Radiol. 2016;81:295.
- Garge S., Keshava SN., Moses V., et al. Radiofrequency ablation of osteoid osteoma in common and technically challenging locations in pediatric population. Indian J Radiol Imaging. 2017;27:88.
- Barnhard R., Raven EE.: Arthroscopic removal of an osteoid osteoma of the acetabulum. Knee Surg Sports Traumatol Arthrosc. 2011;19:1521-1523.


 Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.
Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.