Clinical efficacy of topical nanoemulsion diclofenac gel versus combination of nanoemulsion diclofenac with methyl salicylate in osteoarthritis of knee
2 Department of Anaesthesiology, PDU Medical College, Rajkot, India
Received: 07-Aug-2017 Accepted Date: Sep 06, 2017 ; Published: 11-Sep-2017
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objectives: Topical nanoemulsion diclofenac gels have been introduced on the premise that they act by penetration and accumulation in underlying structures. The other principle of action of topical preparations is “The gate theory of pain” or “Counter irritant principle”. Relative merit of these theories in clinical settings is not known. We compared the clinical efficacy of plain nanoemulsion diclofenac gel with topical preparations that include methyl salicylate, in osteoarthritis of knee. Methods: 200 patients were randomly allocated to either plain diclofenac (1% w/w) nanoemulsion gel (Group D) or diclofenac (1% w/w) with methyl salicylate (10% w/w) (Group DS) or topical methyl salicylate (30% w/w) (Group S) or placebo (petroleum jelly) (Group P). Mean change in VAS score and mean change in WOMAC index was calculated for each group at end of three weeks and compared using Mann-Whitney U test. 50% reduction in VAS score and 50% improvement in modified WOMAC index was considered as “successful treatment” for Numbers Needed to Treat (NNT) analysis. Results: 163 patients were available for final follow up. Both Group D and Group DS showed significant improvement compared to Group P. Group S however did not show significant clinical improvement. Comparing Group D and Group DS, the combination topical preparation showed significant better clinical efficacy. Rate of adverse events in all groups was remarkably low. Conclusion: Combination of topical diclofenac with methyl salicylate was better than topical diclofenac alone, indicating additive effect. Patients who tolerate the counter – irritation may benefit with addition of salicylates to topical diclofenac.
Keywords
Topical, Diclofenac, Methyl Salicylate, Osteoarthritis, Nanoemulsion
Introduction
Topical diclofenac alone and in combination with various agents have been in use in chronic painful conditions like osteoarthritis of knee on a large scale in some parts of the world. In some countries, however these have been approved only recently, that too in limited formulations and dosages [1]. Of late they have been included in pain management protocols in chronic painful conditions like osteoarthritis by several international societies [2] and study groups [3].
Their mechanism of action remains controversial however. Do they act only through systemic route via transdermal absorption? Various new formulations like nanoemulsion gels have been introduced on the premise that they cause inhibition of cyclooxygenase enzyme by penetration and accumulation in underlying musculoskeletal structures [4]. This in principle, may have the advantage of preventing gastrointestinal, cardiac, renal side effects of systemic NSAIDS [1].
The other principle of action of topical preparations is proposed to be “The gate theory of pain” or “Counter irritant principle” [5]. This theory suggests that irritation of sensory nerves of overlying skin offsets pain from underlying muscle or joints [6].
Relative merits and contributions of these theories in clinical settings are not known. Various preparations of plain topical diclofenac are marketed and promoted for being better tolerated as “nonirritant” and having more penetration and absorption. Multiple combination preparations of topical diclofenac with rubefacient’s like methyl salicylate and soothers like menthol, camphor and multitude of other ingredients are also promoted for being more efficacious. Plain diclofenac topical gels do not cause irritation and hence may be more suitable to certain group of patients. Topical salicylates as rubefaciants may help by counter irritation [7] and may also cause cutaneous vasodilatation and hence aid absorption of topical NSAID agent. However, there is very few actual clinical data to support this presumption. Addition of agents like methyl salicylate may cause local side effects and add to unnecessary cost [8].
The aim of this study was to compare the clinical efficacy of plain nanoemulsion diclofenac topical gel with topical preparations that include nanoparticle diclofenac along with methyl salicylate in osteoarthritis of knee through a randomised, placebo controlled, double blind prospective study and thus probe the efficacy and safety of such topical preparations in chronic painful conditions.
Materials and Methods
200 patients meeting inclusion and exclusion criteria were randomly allocated to one of the four groups of plain diclofenac (1% w/w) nanoemulsion gel (Voveran Gel, Novartis Pharma) (Group D) or nanoparticle dicloenac (1% w/w) with methyl salicylate (10% w/w), (Volitra Gel, Ranbaxy pharma) (Group DS) or topical methyl salicylate (30% w/w) (Combiflam cream, Sanofi Aventis) (Group S) or placebo (petroleum jelly) (Group P).
Patients with >50 years age, with chronic knee pain for three months duration or more and radiologically confirmed osteoarthritis of knee (grade 1,2 or 3 according to Kellegren and Lawrence system [9]) were included in the study.
Patients with systemic inflammatory conditions, secondary osteoarthritis, local skin problems, known allergy to any ingredients or those already taking systemic NSAIDS were excluded.
Baseline pain intensity was recorded by Visual Analogue Scale (VAS) Score. Baseline functional and pain score of modified Western Ontario and McMaster Universities Arthritis Index (WOMAC [10]) was also recorded for all patients. We modified the WOMAC index to exclude certain functional activities rendered irrelevant in our group of patients and included some functional activities that were important for local settings and customs (Table 1).
| Activity | Score | ||||
|---|---|---|---|---|---|
| Pain on walking | 0 | 1 | 2 | 3 | 4 |
| Pain on standing | 0 | 1 | 2 | 3 | 4 |
| Pain on going up and down the stairs | 0 | 1 | 2 | 3 | 4 |
| Pain at night | 0 | 1 | 2 | 3 | 4 |
| Pain at rest | 0 | 1 | 2 | 3 | 4 |
| Stiffness in the morning | 0 | 1 | 2 | 3 | 4 |
| Stiffness later in the day after resting | 0 | 1 | 2 | 3 | 4 |
| Difficulty in descending stairs | 0 | 1 | 2 | 3 | 4 |
| Difficulty in ascending stairs | 0 | 1 | 2 | 3 | 4 |
| Difficulty in squatting to the floor | 0 | 1 | 2 | 3 | 4 |
| Difficulty in sitting cross legged | 0 | 1 | 2 | 3 | 4 |
| Difficulty in rising from cross legged | 0 | 1 | 2 | 3 | 4 |
| Difficulty in rising from bed | 0 | 1 | 2 | 3 | 4 |
| Difficulty in lying in bed | 0 | 1 | 2 | 3 | 4 |
| Difficulty in bending to floor | 0 | 1 | 2 | 3 | 4 |
| Difficulty while standing | 0 | 1 | 2 | 3 | 4 |
| Difficulty while walking | 0 | 1 | 2 | 3 | 4 |
| Difficulty in sitting on/off chair | 0 | 1 | 2 | 3 | 4 |
| Difficulty in getting on/off toilet | 0 | 1 | 2 | 3 | 4 |
| Difficulty in getting in/out bath | 0 | 1 | 2 | 3 | 4 |
| Difficulty in commuting by bus/rickshaw | 0 | 1 | 2 | 3 | 4 |
| Difficulty going shopping | 0 | 1 | 2 | 3 | 4 |
| Difficulty in light domestic duties | 0 | 1 | 2 | 3 | 4 |
| Difficulty in heavy domestic duties | 0 | 1 | 2 | 3 | 4 |
| Total Score: 170/96 | |||||
Table 1. Modified Western Ontario and McMaster Universities Arthritis Index.
Randomization was done by generating random numbers and dividing them into four groups. An independent investigator then prepared butter paper packets with doses of topical preparations, each containing single dose of 4 grams and labelled them numerically, to be dispensed in identical containers according to groups previously decided. This ensured double blinding (Flow Chart 1).
Flow Chart 1
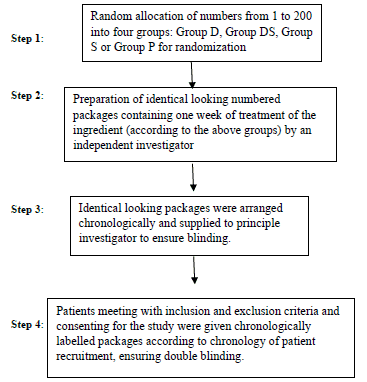
After formal written consent for participation, patients were instructed to gently apply all the content of single packet on one knee three times a day for three weeks. No other forms of local therapy (ice, hot water) were allowed. Oral paracetamol (maximum 2 g/day) was allowed as rescue medication and their uses as well as all local/systemic adverse events were recorded at weekly visits. Final change in VAS score modified WOMAC index was recorded at the end of 3 weeks. Mean change in VAS score (Baseline – Final) and mean change in modified WOMAC index (Baseline– Final) was calculated for each group and compared with placebo group for statistical significance using Mann-Whitney U test. We considered 50% reduction in VAS score and 50% improvement in modified WOMAC index as “successful treatment” for the purpose of generating relative risk ratios and numbers needed to treat (NNT) analysis. These statistical methods have definitive advantage when “normal distribution” of data cannot be assumed as in case of pain scales and functional indices [11,12].
Results
163 patients were available for final follow up, 42 in Group D, 41 in Group DS, 39 in Group S and in 41 Group P. The groups did not differ significantly in age/sex distribution or baseline VAS score or modified WOMAC index. (Table 2). 37 patients were lost to followup (8 from Group D, 9 from group DS, 11 from group S and 9 from group P). Most of these patients didnot want to continue the therapy as they did not find the treatment addressing their symptoms sufficiently.
| Group | Mean Age (years) |
Sex | Mean baseline modified WOMAC index | Mean baseline VAS score |
|---|---|---|---|---|
| Group D | 59.3 ± 5.6 | M-20, F-22 | 62.16 ± 7.97 | 7.95 ± 1.28 |
| Group DS | 60.7 ± 7.1 | M-20, F-21 | 62.09 ± 9.76 | 7.7 ± 1.5 |
| Group S | 60.1 ± 7.02 | M-18, F-21 | 62.3 ± 10.54 | 7.87 ± 1.67 |
| Group P | 60.8 ± 5.8 | M-18, F-23 | 63.04 ± 8.61 | 8.07± 1.23 |
Table 2. Age/sex distribution and baseline VAS score and Modified WOMAC index.
There was significant difference between topical nanoemulsion diclofenac (Group D) and placebo (Group P) in change of VAS score (Z=6.18, p< 0.05) as well as improvement in modified WOMAC index (Z=7.00, p< 0.05). Topical nanoemulsion diclofenac did relieve pain and improve function as compared to placebo in osteoarthritis of knee.
Combination topical preparation (Group DS) also showed reduction in VAS score (z=7.07, p< 0.05) and improvement in WOMAC index (z=7.26, p< 0.05) that was significant compared to placebo. Topical salicylate group (Group S) however failed to show significant reduction in VAS score (Z= -1.45, p=0.14) or WOMAC index (Z= -0.29, p=0.77). Topical methyl salicylate without diclofenac was clinically similar to placebo in our study.
Comparing Group D and Group DS, the combination group showed a significantly greater reduction in VAS score (Z=4.15, p< 0.01) and better improvement in WOMAC index (Z=2.85, p< 0.01) as compared to plain diclofenac. Addition of methyl salicylate to topical nano diclofenac did lead to better clinical efficacy than plain topical nanoemulsion diclofenac in our study.
The relative benefit (RB) of topical diclofenac (Group D) compared to placebo (calculated by comparing percentage of patients experiencing successful treatment for each group) was 0.85 (CI 95% 0.73 to 0.98). Numbers needed to treat (NNT) for group D was 7.02, indicating for every seven patients treated with topical nanoemulsion diclofenac, one would experience successful treatment who would have not have done so with placebo.
The relative benefit (RB) of group DS compared to placebo was 0.62 (CI 95% 0.48 to 0.80). NNT for group DS was 2.7, indicating that for every three patients treated with topical nano diclofenac and methyl salicylate combination, one would experience successful treatment who would not have done so with placebo the relative benefit (RB) of group S compared to placebo was 0.97 (CI 95% 0.89 to 1.06). NNT for group S was 37, indicating that for every 37 patients treated with topical salicylate, one would experience successful treatment that would not have done so with placebo.
Comparing the Relative Benefit of group D and group DS, the relative risk ratio (RRR) was 1.37 (CI 95% 1.02 to 1.84, z value 2.09, p=0.036), significant at p< 0.05 but not at p< 0.01. Addition of methyl salicylate to topical nanoemulsion diclofenac did show better clinical efficacy when assessed with relative risk and number needed to treat analysis (dichotomous data).
Commonest adverse event reported with Group D was local dryness (3 patients,7.14%). While the commonest adverse event reported with Group DS and Group S was bothersome local burning and redness (5 patients, 6.25%). Topical preparations were tolerated well with very low rate of adverse events reported in our study.
The mean use of paracetamol tablets per patient (for 3 weeks) in each group was 12.9 ± 5.14 in Group D, 8.73 ± 5.6 in Group DS, 13 ± 5.71 in Group S and 19.02 ± 5.21 in Group P. All the groups reported significantly decreased paracetamol use as compared to placebo group. Comparing the paracetamol use between group D and Group DS, the combination preparation group had a significantly lower rescue medication use (Z=3.78, p=0.0001).
Discussion
In our study, topical nanoemulsion diclofenac was effective in reducing pain and improving function in osteoarthritis of knee as compared to placebo. Barthel H et al. published similar results in a randomized, controlled trial involving 492 patients and a placebo control in osteoarthritis of knee. They found that at week 12, the topical dicofenac group had significant decreases in mean WOMAC pain, mean WOMAC physical function and mean global rating of disease. Efficacy outcomes significantly favoured topical diclofenac versus vehicle beginning at week 1 in their study. [13] Bookman et al. in their randomized control trial of 248 patients found that the mean change in pain score from baseline to final assessment was significantly greater for the patients who applied the topical diclofenac solution than for those who applied the vehicle-control solution or the placebo solution. For the secondary variables, the topical diclofenac solution also revealed superiority to the vehiclecontrol and placebo solutions, leading to mean changes in physical function, in stiffness and in pain on walking [14].
Topical salicylate without diclofenac was however similar to placebo. Matthews et al. concluded similarly for topical rubefacients after their systemic review of trials involving topical salicylate preparations [8]. Counter irritation pathway alone may be insufficient to cause clinical benefit in chronic painful conditions. Topical preparation with combination of diclofenac and methyl salicylate was better than plain topical diclofenac indicating additive effect of methyl salicylate. Addition of methyl salicylate to topical nano diclofenac improves its clinical efficacy in osteoarthritis of knee.
These preparations were relatively safe with low reported adverse events and did reduce the need of rescue medication in our study. Niethard et al. [15] reported no safety issues concerning adverse events or laboratory values in their research involving 238 patients and topical diclofenac gel in osteoarthritis of knee [16]. Roth and Shainhouse have concluded that topical diclofenac is effective in the treatment of the symptoms of primary osteoarthritis of the knee, with only minor local irritation and no significant systemic adverse events [17].
Further research into concentrations of diclofenac in local muscular tissues and synovial fluid following topical application of diclofenac versus diclofenac and salicylate combination may confirm scientific plausibility and help in understanding the role of counter irritation and local penetration and accumulation of topical NSAIDs.
Conclusion
Patients who tolerate the counter – irritation may benefit with addition of methyl salicylate to topical nanoemulsion diclofenac. These topical preparations reduce the need for systemic/oral analgesics in long standing painful conditions like osteoarthritis. They must be offered judiciously and early in pain management of chronic painful musculoskeletal conditions.
REFERENCES
We thank and acknowledge the efforts by Dr Ravikiran Lagli, second year resident doctor, Department of Orthopaedics for helping in double blinding by preparation of drug packets and numbering them according to random number groups.
REFERENCES
- Derry S., Moore R.A., Rabbie R.: Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Sys Rev. 2012;9:CD007400.
- National Collaborating Center for Chronic Conditions. Osteoarthritis – National Clinical Guidelines for care and management in adults. Royal College of Physicians; London:2008. ISBN:978-1-86016-329-326.
- Marc C., Roy D.A., Karine T.A., et al.: American College of Rheumatology 2012. Recommendations for the use of non-pharmacologic and pharmacologic therapies in osteoarthritis of hand, hip and knee. Arthritis Care and Research. 2012;64 (4):465-474.
- Shin M., Hirkoi I., Eiji K., et al.: Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral application. Br J Clin Pharmacol. 2009; 67:125-129.
- Melzack R., Wall PD.: Pain mechanisms – A new theory. Science. 1965;150:971-979.
- Melzack R., Katz J.: The gate control theory – Reaching for the brain. Pain – Psychological perspectives. Mahwah, N.J. : Lowrence Erlbaum Associates, Publishers; 2004; 0-8058-42993.
- Mason L., Moore R.A., Edwards J.E., et al.: Systemic review of efficacy of topical rubefaciants containing salicylates for the treatment of acute and chronic pain. BMJ. 2004;328:995.
- Matthews P, Derry S, Moore RA, et al.: Topical rubefaciants for acute and chronic pain in adults. Cocharane Database Sys Rev. 2009;8:CD007403.
- Kellgren J.H., Lawrence J.S.: Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494.
- Bellamy N.: WOMAC osteoarthritis index user guide. Version V. Brisbaine, Australia; 2002.
- Sagwick P.: Measuring the benefit of treatment. Numbers needed to treat. BMJ. 2015;350:h2206.
- Moore R.A., Eccleston C., Derry S., et al.: Evidence in chronic pain – establishing best practice in the reporting of systemic reviews. Pain 2010;150:386-389.
- Barthel H.R., Haselwood D., Longley S., et al.: Randomized controlled trail of diclofenac sodium gel in knee osteoarthritis. Semin Arthritis Rheum. 2009;39:203-212.
- Bookman A.M., Williams K.S., Shainhouse J.Z: Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee – a randomized controlled trial. CMAJ. 2004;171:333-1338.
- Ergun H., Kulu D., Kutlay S., et al.: Efficacy and safety of topical nimesulide in the treatment of knee osteoarthritis. Journal of Clinical Rehumatol. 2007;13:251-255.
- Niethard F.U., Gold M.S., Solomon G.S., et al.: Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J Rheumatol. 2005;32:2384-2392.
- Roth S.H., Shainhouse J.Z.: Efficacy and safety of a topical diclofenac solution in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle controlled clinical trial. Arch Intern Med. 2004;164:2017-2023.


 Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.
Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.