Arthritis-A leading cause of bone and joint disruption
Received: 28-Jul-2020, Manuscript No. jotsrr-20-16393; Editor assigned: 30-Jul-2020, Pre QC No. jotsrr-20-16393(PQ); Reviewed: 13-Aug-2020 QC No. jotsrr-20-16393(Q); Revised: 30-Nov-2022, Manuscript No. jotsrr-20-16393(R); Published: 28-Dec-2022
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Arthritis is typically joint inflammation that can generally affect one joint or several joints. There are over 100 different types of arthritis, with varying causes and methods of treatment. Osteoarthritis and rheumatoid arthritis are two of the most common types. Osteoarthritis can also cause inflammatory effects, but over time it often kills the joint cartilage. Rheumatoid arthritis is an autoimmune disorder that is causing symptoms of inflammatory joints throughout the body. Cytokines such as Interleukin 1 (IL-1) alpha, beta or Tumor Necrosis Factor (TNF)-alpha are powerful inductors of Metalloproteinases (MMPs) in synovial rheumatoid fibroblasts and are directly involved in joint destruction. To prevent these various conventional drugs are used like NSAIDs, corticosteroids, cytotoxic drugs etc., but the administration over a longer period may cause side effects. Herbal remedies are now promising approaches to treat mild to severe kinds of inflammation like Curcuma longa, Boswellia etc.
Keywords
Inflammation, arthritis, rheumatoid arthritis, osteoarthritis, tumor necrosis factor, herbal remedies
Introduction
Arthritis is usually a joint inflammation that induces discomfort, pain, stiffness, and swelling. Inflammation is a mechanism in which the white blood cells and immune proteins of the body help to protect us from infection and foreign substances including bacteria and viruses. Arthritis can affect more than one joints. Arthritic disorders can affect the joint tissue as well as the bone, skin, and muscle connective tissue [1].
There are multiple types of arthritis (more than 100) categorized as degenerative, metabolic, and infectious, osteoarthritis and rheumatoid arthritis are the two most common types. Osteoarthritis usually occurs later in life, primarily breaks down joint cartilage over time, and may also induce inflammatory symptoms [2].
Rheumatoid arthritis, an autoimmune disease, induces painful joint pain all over the body. There is an inflammation of synovial membrane and pannus formation that causes joint damage and loss of function. For each patient, the symptoms and complications of rheumatoid arthritis vary. Generally, the symptoms of arthritis develop with time, but they may also appear suddenly. Arthritis most often develops after the age of 65, but can also arise in infants, teenagers, and adults. It is more common in women and in people who are overweighted [3].
Materials and Methods
TYPES OF ARTHRITIS
OSTEOARTHRITIS
This is also known as degenerative joint disease as the most severe form of arthritis The joints of hands, wrists, feet, back, hip, and knees are affected by osteoarthritis. This disease is caused by daily wear and tear of joints and can also occur as a result of an injury.
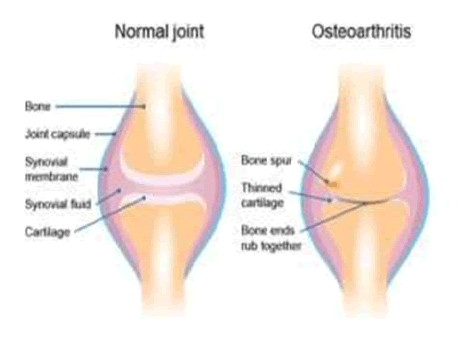
Osteoarthritis begins in the cartilage and the two opposing bones weaken with each other over time, resulting in minor pain during physical activity. Osteoarthritis primarily affects weight-bearing joints like the leg, the neck, and the hip (Figure 1) [4].
RHEUMATOID ARTHRITIS
It is an autoimmune disorder in which the body’s immune system attacks body tissues, not only the tissue of joint but also other parts of the body. In rheumatoid arthritis, two opposite bones are weakened due to damage to the joint lining and cartilage.
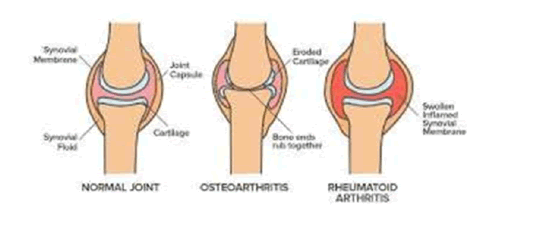
Finger joints, hands, ankles, and elbows are symmetrically affected (appears on both sides of the body) and cause significant deformation if not treated in a few years (Figure 2) [5].
GOUT
In gout, the deposition of uric acid crystals in the joints is triggering inflammation. Usually begins with one, joins, but occurs later in other joints and gets swollen and lacks functionality. Pseudo-gout is a rare type of gout arthritis caused by the development of calcium pyrophosphate rhomboid crystals [6].
SYMPTOMS OF OA and RA
OSTEOARTHRITIS
The principal signs of osteoarthritis are discomfort, physical weakness, and stiffness. It impacts elbows, feet, back, and weight-bearing joints, such as the hips and knees. Heberden's node is a strong bony enlargement of smaller bones like finger bones; they aren't inherently painful but greatly restrict the finger mobility. Osteoarthritis symptoms slowly develop and gradually worsen over a long period [7].
RHEUMATOID ARTHRITIS
Mainly joints are affected by rheumatoid arthritis, but other organs are also affected in more than 15%-20% of cases. Symptoms of rheumatoid arthritis are swelling of the joint (inflammation of synovial membrane), pain, warmth, and stiffness. Polyarthritis is inflammation in multiple joints with time. Small joints of hands, feet, and cervical spine as well as larger joints like the shoulder and knee can also be affected [8].
PATHOGENESIS OF OSTEOARTHRITIS
NORMAL JOINTS
The major constituents of cartilage are water, proteoglycans (composed of protein cores plus chondroitin sulfate and keratin sulfate side chains), and collagen (Predominantly type II) [9]. Collectively, they form the Extracellular Matrix (ECM). Chondrocytes are metabolically active cells that are responsible for the synthesis of the ECM. Proteoglycans provide elasticity of cartilage, and collagen provides tensile strength [10]. Muscles and ligaments provide support and protection, while nerve endings provide proprioceptive information [11]. Cartilage health and function depend on compression (pumping fluid from the cartilage into the joint space and capillaries and venules) and release (allowing cartilage to re-expand, hyper hydrate, and absorb nutrients) [12].
EARLY CHANGES IN OA
The articular cartilage surface becomes irregular with superficial clefts in the tissue with increased chondrocyte proliferation and cluster formation. Increased hydration of the ECM leads to a failure of the elastic restraint of collagen (i.e., weakening of the collagen network) and also alters the proteoglycan distribution. Progression leads to a net decrease in proteoglycans and an increase in the permeability of water [13]. Loss of elasticity and greater permeability of water leads to higher chondrocyte stress and more exposure to degradative enzymes. As the process continues, the articular cartilage has to deepen of the clefts and irregularities, eventually resulting in ulceration and exposure of the bone [14].
LATE CHANGES IN OA
Subchondral osteoblasts increase bone formation, leading to stiffer and less compliant bones. This in turn results in micro-fractures, followed by callus formation, more stiffness, and more micro-fractures. Osteophytes (Outgrowths of bone) are formed, the hallmark of OA. They ultimately cause restricted motion. Subchondral cysts are formed in an attempt to equalize pressure. Gross ulceration of articular cartilage will have focal and then diffuse areas of complete loss of cartilage. In later stages, proteoglycan and keratin sulfate levels in the ECM decrease, as does the length of chondroitin sulfate chains. These changes in concentrations of components within the ECM lead to cartilage resembling the composition of immature cartilage. 20 Soft tissues around the joint are also affected, leading to inflammatory infiltrates in the synovium, greater laxity of ligaments and weakened muscles [15].
PATHOGENESIS OF RHEUMATOID ARTHRITIS
Rheumatoid arthritis begins with synovium. The synovial membrane prepares a cavity or sac around the joint. This cavity contains synovial fluid which lubricates the joints and supplies oxygen to cartilage. In Rheumatoid arthritis, abnormal immune system response is generated which causes the inflammation. Collagen by which cartilage is made up of is generally destroyed resulting in the damaged bone. The destruction of cartilage is accelerated and results in thick synovial tissue formation.
It is due to the accumulation of synovial fluid and immune cells in the synovium [16]. Rheumatoid synovial fibroblasts secrete a large amount of Matrix-Degrading Metalloproteinases (MMPs), which initiate tissue damage by proteolytic degradation of collagens and proteoglycans [17].
MMP-1 degrades structural type I and type II collagen. During type I collagen degradation by MMP-1, Carboxyl-Terminal Telopeptides (CTX) are released and their measurement gives a good estimation of the extracellular matrix degradation. Cytokines, such as Interleukin 1 (IL-1) alpha, beta or Tumor Necrosis Factor (TNF)-alpha are potent inducers of MMPs in rheumatoid synovial fibroblasts and are directly involved in joint destruction [18]. IL-17 is a new cytokine secreted by CD4+ activated memory T cells and produced by RA synovium. The pattern of cellular responses induced by IL-17 is similar to that of IL-1, suggesting that IL-17 may also contribute to joint destruction. Indeed, IL-17 through activation of NF_B, stimulates IL-6, IL-8, G-CSF, and Prostaglandin E2 (PGE2) production by human fibroblasts.
ESTABLISH DIAGNOSIS
• Ultrasound or MRI.
• Erythrocytes Sedimentation Rate (ESR) and C- reactive protein concentration (CRP)
• Rheumatoid factor and anti-citrullinated peptide antibodies
MONITOR DISEASE DAMAGE
• X-rays
• Functional assessment
MONITOR DRUG SAFETY
• Urinalysis
• Full blood count
• Urea, creatinine and liver function tests
Results and Discussion
TREATMENTS
The main objective of the treatment is to reduce the activity of the disease or decrease the inflamed situation with some remission if feasible, reduce joint destruction and ultimately improve the physical condition and quality of life (Table 1) [19-21].
| S.NO | Treatment | Drug used |
|---|---|---|
| 1 | Non-steroidal anti-inflammatory drug | Paracetamol, Opiates, Celecoxib, Ibuprofen, Indomethacin |
| 2 | Disease modified anti-rheumatic drug | Hydroxychloroquine, chloroquine, cyclosporine, Leflunomide,Methotrexate, |
| 3 | Biological therapies | Infliximab, Adalimumab,Cetrolizumab |
| 4 | Corticosteroids | Dexamethasone, Methylprednisolone, Hydrocortisone, Betamethasone |
| 5 | Cytotoxic Drug | Cyclophosphamide, Dacarbazine, Ifosfamide, Doxorubicin |
Table 1. Data showing treatment and drug used.
NATURAL SOURCES TO TREAT ARTHRITIS
TIMES NEW ROMAN CYNODEN DECTYLON
Cynodon dactylon or Bermuda grass is an annual grass cultivated globally, and it is native to warm temperate and tropical regions in particular [22]. Cynodon occurs on nearly all types of soil including in fertile soil [23- 30 ] . For instance the loamy soil . The active constituents found in the plant are flavonoids like apigenin, orientin, luteolin, or vitexin. The plant also contains minerals, carbohydrates, protein, alkaloids, vitamins, palmitic acid, and some essential oil. The species has tremendous medicinal importance and can be applied both externally and
internally. The various study reported an increased level of WBC, ESR, C-Reactive Protein (CRP) and TNFα were substantially suppressed in rats treated with Cynodal dactylon extract and also had a beneficial effect in arthritic joints which were accompanied by a change in bone lesions rather than cartilage lesions [31-38].
ATROPA BELLADONNA
Atropa belladonna Linn is a best known medicinal plant belonging to the solanaceae family, also recognized by the common name of a deadly nightshade and generally known for its exceptional sedative and local pain-relieving function. Atropa genus contains many chemical constituents like flavonoids, phenolic compounds like Alkaloids, alcohols, terpenes, and flavonoids have been identified in this genus.
Different drugs combined with the Atropa extract confirmed stimulating activity in whole blood cultures on the synthesis of the inhibitory cell cytokine TGF-beta. Certain T-lymphocyte pro-inflammatory cells (e.g. IL-1 and TNFα) are excluded during TGF-β development to interrupt the inflammation cycle.
Murraya koengii: Murraya koenigii (Rutaceae) is known in Hindi as the "Curry Patta" and is widely used in the Indian subcontinent and other tropical countries as a spice. Murraya koenigii Methanol Extracts have anti-inflammatory effects. These events can be caused by the heavy concentration of polyphenolic agents, such as alkaloids, flavonoids, tannins, steroids, and phenols. The extract fractions serve as free radical inhibitors or scavengers or may act as primary oxidants, inhibiting the heat-induced denaturation of albumin.
Conclusion
Considerable efforts are needed to improve the perspective of arthritic patients towards a life with less pain and restrictions. Pain is generally treated by using various conventional medicines like NSAID, corticosteroids. The systematic application of DMARD and herbal medicine has resulted in better function and decreased joint damage for patients. However, these medications aren't without side effects or long-term dangers. An appreciation of these risks in both the medical and surgical environments would require better patient treatment.
References
- Ettinger WH, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis: The Fitness Arthritis and Seniors Trial (FAST). Jama 1997;277(1):25-31. [Crossref][Googlescholar][Indexed]
- Reid MC, Shengelia R, Parker SJ, et al. Pharmacologic management of osteoarthritis-related pain in older adults. HSS J 2012;8(2):159-64. [Crossref][Googlescholar][Indexed]
- Schieir O, Tosevski C, Glazier RH, et al. Incident myocardial infarction associated with major types of arthritis in the general population: A systematic review and meta-analysis. Annals Rheumatic Dis 2017;76(8):1396-404. [Crossref][Googlescholar][Indexed]
- Ideguchi H, Ohno S, Hattori H, et al. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arth Res Ther 2006;8(3):R76. [Crossref][Googlescholar][Indexed]
- Chabaud M, Garnero P, Dayer JM, et al. Contribution of Interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 2000;12(7):1092-99. [Crossref][Googlescholar][Indexed]
- Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American college of rheumatology guidelines for management of gout. Part 2: Therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012;64(10):1447-61.[Crossref][Googlescholar][Indexed]
- Arden N, Nevitt MC. Osteoarthritis: Epidemiology. Best Practice Res Clin Rheumatol 2006;20(1):3-25. [Crossref][Googlescholar][Indexed]
- Chaudhari K, Rizvi S, Syed BA. Rheumatoid arthritis: Current Future Trends 2016 [Crossref][Googlescholar][Indexed]
- Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Annals New York Academy Sci 2010;1192(1):230-37. [Crossref][Googlescholar][Indexed]
- Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheumatic Dis Clin North America 1999;25(2):283-98. [Crossref][Googlescholar][Indexed]
- Wojdasiewicz P, Poniatowski LA, Szukiewicz D, et al. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflam 2014. [Crossref][Googlescholar][Indexed]
- Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci 2015;16(11):26035-54. [Crossref][Googlescholar][Indexed]
- Buckwalter JA, anderson DD, Brown TD, et al. The roles of mechanical stresses in the pathogenesis of osteoarthritis: Implications for treatment of joint injuries. Cartilage 2013;4(4):286-94. [Crossref][Googlescholar][Indexed]
- Musumeci G, Szychlinska MA, Mobasher A, et al. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes 2015. [Crossref][Googlescholar][Indexed]
- Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatol 2004;43(suppl_3):iii2-9. [Crossref][Googlescholar][Indexed]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New Engl J Med 2011;365(23):2205-19. [Crossref][Googlescholar][Indexed]
- Angelott F, Parma A, Cafaro G, et al. One year in review 2017: Pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol 2017;35(3):368-78. [Crossref][Googlescholar][Indexed]
- Deane KD, Norris JM, Holers VM, et al. Preclinical rheumatoid arthritis: Identification, evaluation, and future directions for investigation. Rheumatic Dis Clin 2010;36(2):213-41. [Crossref][Googlescholar][Indexed]
- Kikuchi M, Matsuura K, Matsumoto Y, et al. Bibliographical investigation of complementary alternative medicines for osteoarthritis and rheumatoid arthritis. Geriatrics Gerontol Int 9(1):29-40. [Crossref][Googlescholar][Indexed]
- Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Therapy 2013;15(S3):S2. [Crossref][Googlescholar][Indexed]
- van Laar M, Pergolizzi Jr JV, Mellinghoff HU, et al. Pain treatment in arthritis-related pain: Beyond NSAIDs. The Open Rheumatol J 2012;6:320. [Crossref][Googlescholar][Indexed]
- Scott C, Meiorin S, Filocamo G, et al. A reappraisal of intra-articular corticosteroid therapy in juvenile idiopathic arthritis. Clinical Exper Rheumatol 2010;28(5):774. [Crossref][Googlescholar][Indexed]
- Malhotra S, Welling MN, Mantri SB, et al. In vitro and invivo antioxidant, cytotoxic, and anti‐chronic inflammatory arthritic effect of selenium nanoparticles. J Biomed Materials Res Part B: Applied Biomaterial 2016;104(5):993-1003. [Crossref][Googlescholar][Indexed]
- Schneeweiss S, Setoguchi S, Weinblatt ME,et al. Anti–Tumor Necrosis Factor α therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis. Arthritis Rheumatism 2007;56(6):1754-64. [Crossref][Googlescholar][Indexed]
- Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytotherapy Res 2012;26(11):1719-25. [Crossref][Googlescholar][Indexed]
- Arora R, Kuhad A, Kaur IP, et al. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant‐induced arthritis in rats. Euro J Pain 2015;19(7):940-52. [Crossref][Googlescholar][Indexed]
- Nikam TD, Ghorpade RP, Nitnaware KM, et al. Micropropagation and non-steroidal anti-inflammatory and anti-arthritic agent boswellic acid production in callus cultures of Boswellia serrata Roxb. Physiol Mol Biol Plants 2013;19(1):105-16. [Crossref][Googlescholar][Indexed]
- Suva MA, Kheni DB, Sureja VP. Aflapin: A novel and selective 5-lipoxygenase inhibitor for arthritis management. Indian J Pain 2018;32(1):16. [Crossref][Googlescholar][Indexed]
- Chaturvedi D, Dwivedi PK, Chaturvedi AK, et al. Semisynthetic hybrids of boswellic acids: A novel class of potential anti-inflammatory and anti-arthritic agents. Med Chem Res 2015;24(7):2799-812. [Crossref][Googlescholar][Indexed]
- Arya D, Meena M, Grover N, et al. In vitro anti-inflammatory and anti-arthritic activity in methanolic extract of cocculus hirsutus (l.) Diels. In vivo and in vitro. Int J Pharma Sci Res 2014;5(5):1957. [Crossref][Googlescholar][Indexed]
- Rahmatullah M, Khatun Z, Hasan A, et al. Survey and scientific evaluation of medicinal plants used by the Pahan and Teli tribal communities of Natore district, Bangladesh. African J Trad, Compl Alternative Med 2012;9(3):366-73. [Crossref][Googlescholar][Indexed]
- de Wet H, Van Wyk BE. An ethnobotanical survey of southern African Menispermaceae. South African J Botany 2008;74(1):2-9. [Crossref][Googlescholar][Indexed]
- Su S, Hua Y, Wang Y,et al. Evaluation of the anti-inflammatory and analgesic properties of individual and combined extracts from Commiphora myrrha, and Boswellia carterii. J Ethnopharmacol 2012;139(2):649-56. [Crossref][Googlescholar][Indexed]
- Ammar NM, El-Hawary SS, Mahdy AA, et al. Phytochemical study of the biologically active fractions of the oleo-gum-resins of Boswellia carteri and Commiphora myrrha. Advances Environ Biol 2013;2573-84. [Crossref][Googlescholar][Indexed]
- Ashokkumar K, Selvaraj K, Muthukrishnan SD. Cynodon dactylon (L.) Pers.: An updated review of its phytochemistry and pharmacology. J Med Plants Res 2013;7(48):3477-83. [Googlescholar][Indexed]
- Sindhu G, Ratheesh M, Shyni GL,et al. Inhibitory effects of Cynodon dactylon L. on inflammation and oxidative stress in adjuvant treated rats. Immunopharmacol Immunotoxicol 2009;31(4):647-53. [Crossref][Googlescholar][Indexed]
- Akbar S. Atropa belladonna L.(Solanaceae). In Handbook of 200 Medicinal Plants. Springer, Cham 2020;373-9. [Crossref][Googlescholar][Indexed] Sriram K, Vishnpriya V, Ponnulakshmi R, et al. Anti-inflammatory activity of Murraya koenigii An in vitro study. Drug Invention Today 2019;11(10).[Googlescholar]
- Darvekar VM, Patil VR, Choudhari AB. Anti-inflammatory activity of Murraya koenigii Spreng on experimental animals. J Nat Prod Plant Resour 2011;1(1):65-9. [Googlescholar]


 Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.
Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.